Health Care R&D Funding to Increase with 21st Century Cures Act
Earlier this month, the US Congress and President Obama passed the 21st Century Cures Act, a law addressing the reformation of certain health care policies, as well as the R&D and approval process for experimental medicines. Initiated by Energy and Commerce Committee Chairman Fred Upton and Representative Diana DeGette in spring 2014, the bill is the culmination of research and analysis regarding the medicinal treatment cycle of discovery, development, and delivery. Over the course of a year, the 21st Century Cures Initiative Committee conferred with health care patients, providers, regulatory agents, researchers and white papers, and conducted eight hearings and over 12 roundtables in representative districts around the nation.
The Act calls for provisions to modernize the current health care system through interoperability, which involves various health care networks efficiently and securely exchanging information and patient data; improving clinical trials by incorporating more of the patient’s feedback into the FDA’s drug and device approval process; amending the drug and device approval process itself to include submissions of qualified scientific and medical developments to physicians, insurance companies and researchers to enhance patient care, which will streamline the medicine-approval process; streamlining regulatory procedures for companies developing new vaccines and treatments; and encouraging pharmaceutical companies to utilize modern manufacturing technologies in the US instead of going overseas.
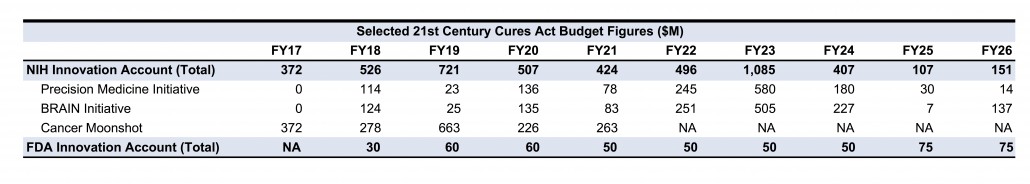
Also included in the Act is $1.8 billion, distributed over the next five years, for new resources for the Cancer Moonshot initiative. In addition, approximately $3 billion has been allocated for biomedical research initiatives, such as the BRAIN (Brain Research through Advancing Innovative Neurotechnologies) and Precision Medicine programs, which received total funding of $1.6 billion and $1.4 billion, respectively, from the 10-year period of FY17 to FY26.
The NIH reauthorization includes $34.8 billion for FY18, $35.6 billion for FY19 and $36.5 billion for FY20. The Intramural Loan Repayment Program, which is designed to repay up to $35,000 of a researcher’s education debts, was amended to now pay up to $50,000 of qualified education debts. For the R&D and treatment of neurological diseases, $5 million for each fiscal year from 2018 to 2022 was appropriated, while $15 million was allocated to health care information technology in the form of the Electronic Health Reporting program.
Additionally detailed in the law was the coordination between the NIH and FDA for awarding grants and clinical research contracts to accelerate regenerative medicine using stem cells, including autologous stem cells. The collaborative award was appropriated $30 million, to be distributed equally over the three-year period of FY18–FY20.