Automating Rapid and Accurate Identification of the N-Glycans of a Recombinant Human Erythropoietin (rhEPO) Expressed by a Chinese hamster Ovary (CHO) Cell Line using Tandem MS/MS Data
March 27, 2018
Biopharmaceuticals represent the fastest growing sector of the pharmaceutical industry, mainly driven by a rapid expansion in the manufacture of recombinant protein-based drugs [1]. The majority of the therapeutic proteins are glycoproteins (monoclonal antibodies, blood proteins, growth factors, vaccines, fusion proteins) and hence, glycosylation is the most prominent post-translational modifications occurring on these protein drugs.
Chinese hamster ovary (CHO) cells are the most commonly used mammalian hosts for industrial production of recombinant protein therapeutics [2] (e.g., recombinant human erythropoietin (rhEPO), a heavily glycosylated protein with three N-glycosylation sites expressed by a Chinese Hamster Ovary (CHO) cell line).
Mass spectrometry (MS), either direct infusion or coupled to chromatographic methods, are routinely used to elucidate complex glycan structures. SimGlycan software predicts and scores candidate glycans for each MS/MS scan by creating a list of candidate structures using the precursor m/z and then comparing the in-silico fragment ions of each candidate against the observed product ions [3].
To facilitate rapid identification of N-glycans of rhEPO using tandem MS data, we have created a database of glycans curated from published literature. The MS/MS database search can be performed only on the specific database instead of searching the global SimGlycan database, thereby increasing the confidence of the IDs.
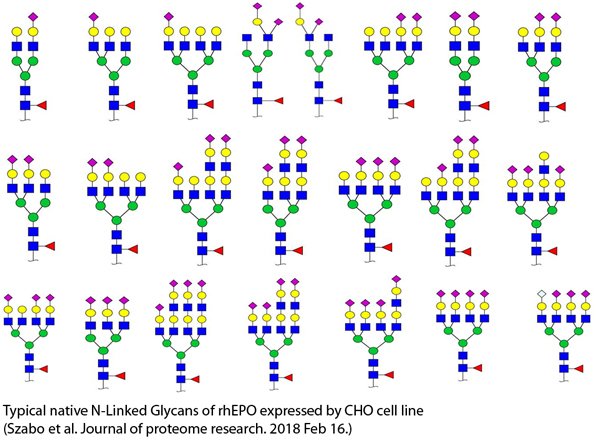
List of N-Glycans of CHO Cell Lines
Future Direction: The current database does not contain glycans with modifications such as phosphorylation, sulfation, and sialic acids with possible O-Acetylation. Typically, rhEPO contains large number of tetraantennary sialylated glycans wherein sialic acids (mostly N-acetylneuraminic acid, Neu5Ac) are attached to galactoses via α-2,3 linkages. O-Acetylated sialic acid and a small amount of N-glycolylneuraminic acid (Neu5Gc) modified glycans are also present on rhEPO. Other modifications on rhEPO glycans are (a) phosphorylation – mostly high mannose glycans at low, but not negligible levels, and mannose 6-phosphate; and (b) sulfation [4]. We are creating a database containing curated glycan structures with various modifications that were verified using the following workflows [4]: (1) hydrophilic interaction chromatography (HILIC) coupled to a Q Exactive Orbitrap mass spectrometer, and data dependent HCD fragmentation; and (2) High performance anion exchange (HPAE) chromatography coupled to a Q Exactive Orbitrap mass spectrometer, and data dependent HCD fragmentation.