Medicare Gives Green Light to NGS IVDs
In what is viewed as a step forward for NGS-based clinical testing, the US Centers for Medicare & Medicaid Services (CMS) announced this month a final National Coverage Determination (NCD) for FDA-approved NGS tests for advanced cancer (defined as either recurrent, relapsed, refractory, metastatic, or advanced stages III or IV) patients as long as certain criteria are met. An NCD decides what services will be paid for by the government’s national Medicare program. Medicare covers roughly 15% of the US population, according to AARP. The NCD was specifically issued for Foundation Medicine’s FoundationOne CDx (Companion Diagnostics) test for solid tumors.
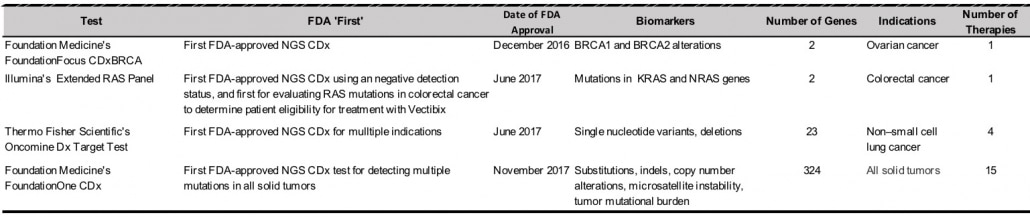
With the CMS’ first reimbursement approval of an NGS IVD test, and the policy it appears to set, NGS diagnostic testing overcomes another barrier to routine use. Also, the NCD builds upon the momentum created by a number of FDA-approval “firsts” for NGS IVDs over the last year and a half (see table below). NGS CDx tests have been pioneers in this regard. In total, of the 5 FDA-approved NGS tests, 4 are CDx tests. The approvals also suggest the FDA’s growing regulatory acceptance of the single test–multiple drug and single test–multiple determination models enabled by NGS, in addition to the traditional single test–single drug model for CDx.
The NCD is also important, according to commentators, in that it revises an earlier draft that had put greater restrictions on coverage, and thus allays some concerns about the CMS’ approach, particularly in regard to local Medicare (private contractors that process Medicare claims) coverage and disease criteria for coverage. As R. Bruce Williams, MD, FCAP, president of the College of American Pathologists, put it in a press release, “While the final NCD nationally covers only approved or US Food and Drug Administration (FDA)–cleared tests using NGS, the final determination leaves the local Medicare Administrative Contractor with the discretion to cover all other tests as long as specific patient criteria are met. The expanded criteria include recurrent, relapsed, refractory, metastatic, and advanced stages III or stages IV of cancer.”
Impact?
However, the NCD’s ultimate impact on FDA-approved NGS tests is still uncertain. “It is too early to say what effect the NCD will have on adoption of FDA-approved NGS tests,” said Richard L. Schilsky, MD, FACP, FASCO, FSCT, chief medical officer of the American Society of Clinical Oncology (ASCO). “In general, ASCO supports regulatory oversight of these complex tests, but appropriate oversight should not impede access to medically appropriate NGS testing.”
For FDA-approved NGS CDx test and sequencer manufacturers Illumina and Thermo Fisher Scientific, the NCD was well received. Joydeep Goswami, PhD, president of Clinical Next Generation Sequencing and Oncology for Thermo Fisher, thinks the NCD will positively impact NGS IVD testing in general. “We expect this CMS decision to encourage the development of more NGS-based tests and greater adoption of these tests by labs to help patients,” he said. “We are also hopeful that the FDA will continue to streamline requirements to help these tests to get to market faster and more cost effectively.”
Illumina also greeted the NCD announcement with enthusiasm, highlighting the implications for CMS reimbursement for all FDA-approved NGS IVDs. “[B]ased on the language in the final NCD, coverage for FDA-approved, NGS-based, CDx assays in advanced cancers will be automatic in the future,” said Garrett Hampton, PhD, executive vice president, Clinical Genomics, for Illumina. “Thus, this decision opens up a clear pathway for reimbursement of CDx assays that identify patients for novel targeted therapies, as well as for CDx assays measuring tumor mutational burden for Immuno-Oncology (IO) indications, and combinations of targeted therapies with IO therapies.” In addition to CDx for standard treatments, the FoundationOne CDx test also includes biomarkers for targeted oncology therapies, such as IO. “This NCD comes at a time when the market is poised to leverage gene panel sequencing to support emerging targeted and IO therapies,” said Dr. Hampton.
The NCS is especially significant to both companies as each are working to establish their newly FDA-approved NGS IVDs and roll out future such tests. Illumina’s Extended RAS CDx panel for metastatic colorectal cancer, which gained FDA approval and was launched last year, is the company’s first PreMarket Approval (PMA) and first CDx test. Illumina’s NGS test previously approved by the FDA was approved under 510(k) clearance, a less rigorous standard than PMA as it only requires substantial equivalence to a previously approved device.
The Extended RAS Panel is also important as a product for FDA-approved NGS LDTs, according to Dr. Hampton. “Notably, a key aspect of the Illumina NGS-based CDx test is that the Extended RAS Panel on the MiSeqDx System enables labs to implement an in-house solution for precision oncology, and signifies that NGS has reached a milestone as a clinical diagnostic platform to aid therapeutic decision-making in oncology,” he told IBO. Foundation Medicine’s test must be performed at its own labs. Illumina continues to develop NGS CDx tests and has publicly announced partnerships with drug makers Amgen, AstraZeneca and Merck Serono for NGS CDx development.
Illumina’s plans include eventual FDA-approval of its TruSight panels, which are currently available as RUO panels, each using multiple markers to test for specific disease types. Illumina’s RUO TruSight Tumor 170 test includes biomarkers for effectiveness of IO treatments. “At JP Morgan this year, we announced our intention to extend the TruSight menu to include a 500-gene panel, which we intend to bring to market as an IVD long term to assess tumor mutation burden for stratification of checkpoint inhibitors,” explained Dr. Hampton.
For Thermo Fisher Scientific, which is developing additional indications for its FDA-approved Oncomine Dx Target Test for CDx, the first NGS-based test to receive FDA approval for Non-Small Cell Lung Cancer (NSCLC), the NCD guarantees CMS reimbursement for future extensions of the test. “Given that CMS had determined to cover NGS tests that are approved by FDA, expansion of the Oncomine Dx Target Test with additional genes/markers for other indications/drugs in cancer would automatically be covered by CMS once these have received FDA approval via a supplemental PMA,” explained Dr. Goswami.
In addition, it affects CDx tests yet to be introduced by the company. Last year, Thermo Fisher announced agreements with Argios Pharmaceuticals to develop a Oncomine-based CDx for markers of advanced IDH1m positive cholangiocarcinoma (bile duct cancer) and with Blueprint Medicines to develop an Oncomine Dx test for new markers of NSCLC. In addition, the company has publicly announced CDx partnerships with GlaxoSmithKline, Novartis and Pfizer. And the Oncomine family of tests are also expanding beyond CDx to IO. Earlier this year, Thermo Fisher launched the RUO-only Oncomine Immune Response Research Assay IO for clinical research trials.
Private Payer Reimbursement?
Another question is the impact of the final NCD on private payer reimbursement for FDA-approved NGS IVDs. Describing the situation at it stands now, Dr. Schilsky told IBO, “The current environment is in flux and so this question is difficult to answer. However, ASCO was supportive of this NCD in general because it represented a first step toward securing consistent nationwide reimbursement for medically appropriate NGS testing.”
Both Illumina and Thermo Fisher also view the NCS as progress in this regard. “For non-Medicare plans, commercial payers often follow the lead of CMS in making coverage decisions—in particular for FDA-approved products—and we hope to see that happen with the implementation of this NCD,” stated Dr. Hampton. “While private payers make their coverage decisions independently, we do expect that the recognition provided by CMS to FDA-approved NGS tests will impact their decisions on Oncomine Dx coverage favorably,” explained Dr. Goswami. Thermo Fisher has announced coverage for the FDA-approved Oncomine Dx by a number of large private insurance providers, including Aetna and UnitedHealthcare.
Although public and private reimbursement is just one issue affecting NGS IVD test usage, it cannot be taken for granted. Asked about the future developments required to grow the use of FDA-approved NGS CDx tests, Dr. Schilsky commented, “That is a complex question. The issue is less about ‘growing the use’ of these tests and more about defining their appropriate use and clinical utility in the care of patients with cancer.” However, reimbursement is an important consideration. “Consistent reimbursement along the lines of this NCD is certainly necessary, as well as a well-defined regulatory pathway to market.”
But with NGS being a new diagnostic technology, the NCD’s effect in this regard is especially unclear. “Historically, private payers tend to follow CMS’ lead more often than not, but this field is simply too new to say anything with certainty,” stated Dr. Schilsky. “ASCO would hope that private payers will provide coverage for well-validated tests that have demonstrable positive impact on patient outcomes.”
Along these lines, the NCD has other ramifications. Unlike the earlier draft, the final NCD does not necessitate Coverage with Evidence Development, which requires additional collection of clinical data to determine effectiveness, for non-FDA-approved test or tests without an FDA-approved CDx for the specific type of cancer. “ASCO would also stress the need for ongoing evidence development to improve the reliability of these tests,” stated Dr. Schilsky. “The elimination of Coverage with Evidence Development as a means of speeding insight into a critical/complex component of treatment overlooks an important opportunity to accelerate and improve insight into their application and demonstration of their utility.”
The Future?
Helped by both FDA approvals and public and private payer reimbursement policies, it is clearly just the beginning for the use of NGS IVD tests. Both Illumina and Thermo Fisher Scientific are moving forward to expand the market with new testing modalities. With NGS CDx tests now FDA and CMS approved, and progressing for CDx IO testing, NGS liquid biopsy testing is set to follow. “Liquid biopsy is likely to become the next frontier as evidence emerges demonstrating the relative performance of genomic testing from tissue and plasma,” explained Dr. Hampton. As Dr. Goswami told IBO, “These developments should lead to the rapid extension of NGS-based FDA approved tests to liquid biopsy and immuno-oncology-based tests that help doctors better analysis approaches to better understand and address cancer.”