MS and NGS Play Bigger Roles at AACC 2017
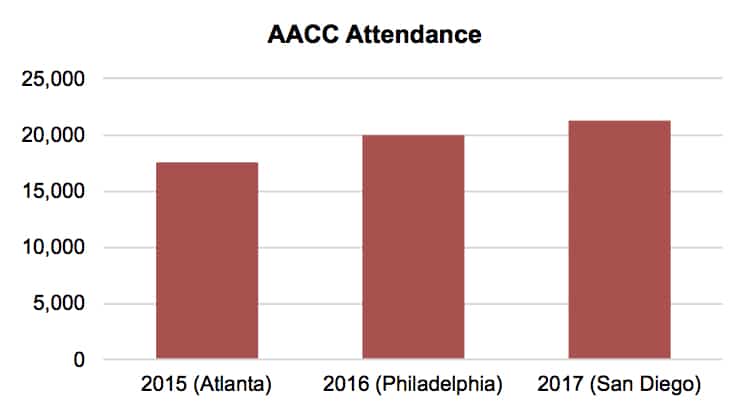
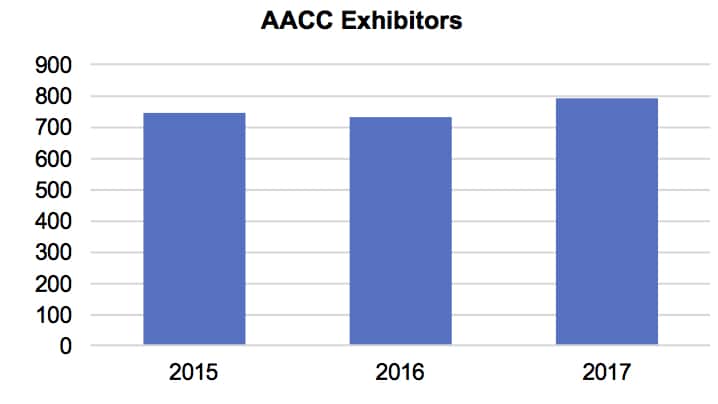
The American Association of Clinical Chemistry’s (AACC) 69th Annual Scientific Meeting and & Clinical Lab Expo took place July 30–August 3 in San Diego, California. The show’s attendance rose 7.9% to over 21,300. The exhibition hosted over 750 exhibitors (see table below).
Among the exhibitors were leading triple quadruople MS companies, including Agilent Technologies, SCIEX, Shimadzu, Thermo Fisher Scientific and Waters. Although Bruker was not at the show, bioMérieux, another MALDI-TOF firm serving the clinical market, exhibited.
At the show, SCIEX formally launched the SCIEX Topaz System, an LC/MS system for clinical diagnostics, and the integrated ClearCore MD software (see SCIEX Diagnostics Discusses Its New Clinical LC/MS System). It follows the company’s May launch of the first FDA-approved Vitamin D 200M assay for quantitative determination of total 25-hydroxyvitamin D through measurements of 25-hydroxyvitamin D3 and D2 in human serum. The test is designed for exclusive use on the Topaz System for assessment of adult patients for Vitamin D sufficiency. The Topaz, a Class II medical device, also runs LDTs. The system’s ClearCore MD software is a completely new platform designed for non-MS experts.
As a sign of the increasing competition in clinical MS, but taking a closed system approach, Thermo Fisher Scientific was also at the show, providing demonstrations of the Thermo Scientific Cascadion SM Clinical Analyzer (see IBO 6/15/17). Consumables for the system include specialized pipettes, LC cartridges and special sample tubes for sample preparation automation. The system is expected to be commercialized next year with kits to follow.
Waters showcased its Class I MS systems at the show. The company sells three versions of its tandem quadrupole MS systems in the clinical market. The company’s 510(k) approved MassTrak Immunosuppressant Kit for monitoring tacrolimus via MS was introduced in 2007. For the European market, the company offers the CE-marked MassTrak Vitamin D Solution, and MassTrak Immunosuppressants XE Kit for quantifying tacrolimus and everolimus, in addition to the MassTrak Immunosuppressant Kit. Waters also provides both IVD and research columns for the systems, as well as sample preparation kits and reference materials.
The clinical MS market also includes PerkinElmer, which provides kits for neonatal screening. Linh Hoang, vice president of Neonatal Screening at PerkinElmer, told IBO that the company screens more than 39 million babies per year. The business is expanding in China, where more than 90% of newborns are now screened. The opportunity is also growing in India. “Newborn screening pilot programs in the public sector (using PerkinElmer instrumentation and kits) have demonstrated the value of newborn screening,” he said. PerkinElmer provides the testing as a service at its clinical labs in Suzhou, China and Chennai, India.
Although not the focus of the show, NGS and MS were represented in the program. In a Tuesday morning session, “Beyond Sequencing: New Frontiers in Genomics,” Jay Shendure, MD, PhD, of the University of Washington, Seattle, discussed his lab’s work in using DNA barcodes in single-cells to create an organism development tree for zebrafish. As part of the work, the lab is also exploring how cell types are related to one another. Dr. Shendure noted that the main challenge for single-cell analysis is isolating cells.
A Tuesday afternoon symposia focused on MALDI-TOF in the clinic. Entitled “MALDI-TOF Mass Spectrometry: Not Just for Clinical Microbiology Labs Anymore,” it was standing room–only for a presentation by Mari DeMarco, PhD, of the University of British Columbia. In her talk, she discussed the use of MALDI-TOF for epitope mapping to determine immunoassay reactivity. The research was conducted based on the testing of a patient for AdrenoCorticoTropic Hormone (ACTH) levels. Using immuno-MALDI-TOF MS, Dr. DeMarco mapped the epitopes of the ACTH present in the patient to determine the two antibodies in a Roche immunoassay kit to which they bind. The characterization of the ACTH fragment mixtures were used to distinguish the antibody’s binding site and develop an internal standard for LC/MS/MS. She ultimately characterized the plasma ACHT-ome, identifying discrepancies in how the immunoassay worked. As she told the audience, “Don’t be scared of MALDI-TOF.”
In the second presentation, Tony Y. Hu, PhD, of Arizona State University discussed the use of MALDI-TOF to quantitate protein biomarkers using nanoparticles. He highlighted the advantages of MALDI-TOF, including speed, ease of use, high throughput and high sensitivity. His research targets the development of diagnostic tests for TB based on biomarkers. Specifically, he has created nanopore silicon porous particles to detect MTb-specific antigen markers in blood samples.
Present at the show were a number of vendors known for their research tools that are now growing their diagnostics businesses, including Abcam, Bio-Techne, Promega and TTP. Speaking with IBO, Abcam discussed its expanding business for providing antibodies for clinical applications, especially companion diagnostics. The company is partnering with pharmaceutical companies to develop both custom antibodies and antibodies that are subsequently commercialized, such as anti-PD-L1 primary antibody [28-8] and others. Other clinical applications for the company’s antibodies include clinical trials.
AACC 2018 will be held July 29–August 2 in Chicago, Illinois.