Collaborative Research with PeptiStar Inc., Supporting the Development of Nucleic Acid-Based Therapeutics Release of Software for Oligonucleotide Characterization, LabSolutions Insight Biologics
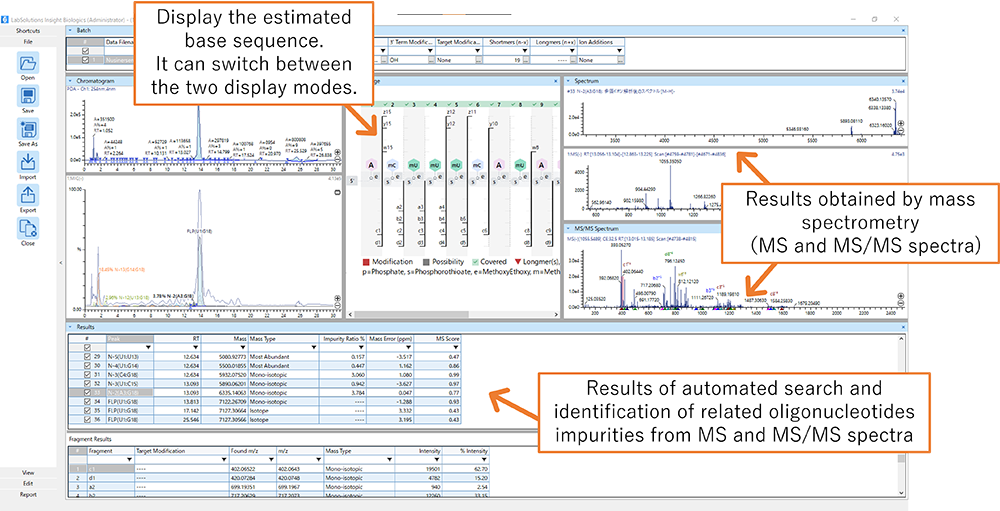
On June 5, Shimadzu Corporation will release LabSolutions Insight Biologics, a software for oligonucleotide characterization for the LCMS-9030 and LCMS-9050 high-performance liquid chromatograph quadrupole time-of-flight (Q-TOF) mass spectrometers. This software automatically conducts both measurement and analysis when characterizing oligonucleotides, reducing the analysis time to 1/10 or less compared to conventional practices. Equipped with functions such as display of molecule structural formulas, suggested calculation methods for component ratios, and impurity estimation technology (patent pending), the software helps increase efficiency of nucleic acid-based therapeutic research.
Photo: LabSolutions Insight Biologics Software Window
Nucleic acid-based therapeutics are a type of biopharmaceutical consisting of DNA and RNA. They have minimal side effects and are expected to be applicable to diseases that have been difficult to treat. They are seen as “the third promising type of pharmaceutical” after small molecule pharmaceuticals and antibody pharmaceuticals. Oligonucleotides, which are the pharmaceutical ingredients in nucleic acid-based therapeutics, are generally formed by chemical synthesis. However, unintended impurities can be produced in the process. Safety confirmations and evaluations are required, but because the molecular weights of principal components are similar, precise weight measurements are needed, making characterization difficult.
LabSolutions Insight Biologics was developed based on the results of collaborative research with PeptiStar Inc., a contract development and manufacturing organization (CDMO) for peptide pharmaceutical*1 and nucleic acid-based therapeutic ingredients. When evaluating the characterization process, samples which mimicked the nucleic acid-based therapeutics provided by PeptiStar were used, so highly precise characterization was achieved. The software has been made user-friendly by developing it based on the actual workflow required for this research, from research and development to pharmaceutical ingredient manufacturing and impurity confirmation.
Features
1. Smooth Oligonucleotide Characterization
Checking for impurities produced in the process of synthesizing oligonucleotides takes a great deal of time and labor. Shimadzu has developed a simple and rapid characterization workflow using data from the collaborative research with PeptiStar. A comprehensive search for the principal components and their related impurities is performed automatically by the MS and MS/MS spectra*2, reducing the time needed for characterization to 1/10 or less of the conventional amount required.
2. Easy Workflow for Anyone
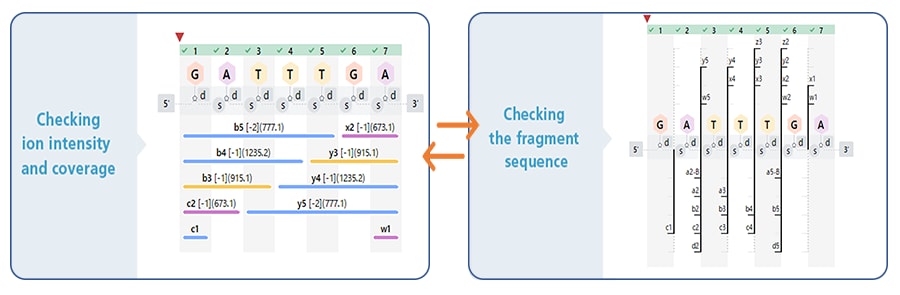
With our proprietary software functions the workflow is easy for any operator to follow. Normally, the nucleotide sequence for the principal components must be entered prior to oligonucleotide sequence measurement, which is a complicated step prone to error. However, with a function that displays the nucleotide sequence information as a structural formula in real time, the principal components logged can be checked visually. In addition, the software is equipped with a function for switching the display between full mode (figure below on the left) for checking the accuracy of the base sequence*3, and branch mode (figure below on the right) for checking the positions where impurities tend to be produced. By checking results with these two modes, the estimated sequence can be more accurately evaluated.
3. Estimating the Base Sequences Required to Identify Impurities
When identifying oligonucleotide acid analogs, checking for impurities with similar molecular weights and base sequences is a difficult and complicated task. This software uses our proprietary fragment spectrum method (patent pending) to estimate base sequences with high accuracy even from among many similar molecules. Efficiently consolidating multiple MS/MS spectra simplifies spectral information checks and limits the impact of variability in each measurement.
- *1: These are pharmaceuticals in which the principal components are peptides consisting of 2 to 50 bonded amino acids. They are characterized by a wide range of action and fewer side effects.
- *2: This data is obtained from mass spectrometry results. Mass is plotted on the vertical axis, and the detection intensity is plotted on the horizontal axis. It contains a lot of molecular weight information about principal components and residues.
- *3: These are the units for the configuration of oligonucleotides. They consist of four types. The sequence must be checked to identify the impurities.
For more details, visit
LabSolutions Insight Biologics