Sequencing in Clinical Labs: Good News for the NGS Sample Prep Market
The medical field is evolving as treatment models shift away from the traditional “one size fits all” approach, which treats the “average” patient. Precision medicine, also called personalized medicine, is gradually becoming the new model for medical diagnosis and treatment. Under this model, treatments are tailored to the individual characteristics of each patient and their disease, taking into account underlying genetics and biomarkers presented by the disease that could serve as therapeutic targets. To this end, the pharmaceutical and biotech sector has rushed to develop highly targeted therapeutics, working closely with collaborators to characterize and validate treatment targets.
Sequencing has been the driving technology to make this possible, both in terms of diagnosis and the development of targeted therapies. Next generation sequencing (NGS) has had an enormous impact on life science and medicine since its commercialization over a decade ago. As the technology has matured and become more accessible and affordable, the use of NGS has expanded beyond the lab to the medical diagnostics space. NGS has enabled the accelerated understanding of the genetic basis of many diseases and the development of advanced, targeted therapeutics. Patients are now beginning to directly benefit from these advancements through precision medicine.
The advancement of precision medicine has been supported by governments around the world. In 2015, the US launched the Precision Medicine Initiative, renamed All of Us, which aims to “understand how a person’s genetics, environment and lifestyle can help determine the best approach to prevent or treat disease.” China launched its Precision Medicine Initiative in 2016, with $9.2 billion in funding over 15 years. These projects, and similar projects underway around the globe, involve huge sequencing components, which will combinedly generate data for millions of human genomes.
Thus far, the US has led market adoption of precision medicine, with clinical labs offering sequencing services for tissue and liquid biopsy samples, prenatal testing, and others. The FDA has cleared several sequencing assays for molecular diagnostics, but the majority of assays are conducted as laboratory developed tests (LDTs). Demand is rapidly growing, however, as third-party payors become more willing to accept sequencing assays.
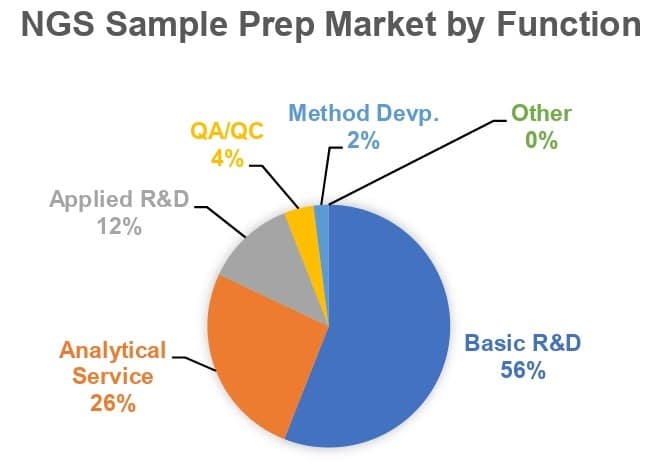
The increasingly routine use of sequencing in clinical settings is creating tremendous opportunities for suppliers of sequencing instrumentation and consumables, including those required for preanalytical preparation of samples from biological materials. In fact, analytical services for clinical applications were the second leading function of NGS sample prep in 2018 (see graph below), and will drive double-digit growth for the NGS sample preparation market over the next five years.
In the clinical lab, NGS, like all assays, is subject to validation at all steps to recognize and address variables that may affect analysis. This is very important even for preanalytical sample preparation, since variables will carry forward to affect the sequencing and post-sequencing bioinformatics stages. Variables that must be considered include how the sample was collected and fixed and sample quality, both of which will affect the selection of an appropriate library preparation kit.
For instance, the standard for preservation of tissue biopsies samples is formalin-fixed paraffin-embedded (FFPE). But formalin fixation causes crosslinking, resulting in artifacts in downstream analysis. Sample quality includes assessment of the pathological composition of the sample, since tissue biopsies may contain a mixture of normal tissue and tumor cells in varying proportions. Using an appropriate library preparation kit can mitigate some of these variable, such as using a kit designed for FFPE samples and optimized for low-frequency variants.
A recently published report, “Sample Preparation for Next Generation Sequencing” from SDi, IBO’s publisher, provides an in-depth analysis of how precision medicine and other applications are shaping demand for NGS sample prep techniques. NGS sample prep generated $1.8 billion in revenues in 2018, encompassing nucleic acid extraction from biological materials, fragmentation of nucleic acids into smaller pieces and the preparation of sequencing libraries.
The fast growth of NGS applications for both clinical and diagnostics applications promise a greater need for NGS sample prep products, as well as an ongoing launch of new products from leading companies in the market, such as Illumina, New England Biolabs and QIAGEN.