A Step Forward for Point-of-Care Testing: COVID-19 Tests Gain FDA EUA Approval
Marking another new step forward in more effective COVID-19 testing, as of April 2, the US FDA had approved three molecular point-of-care (POC) tests for COVID-19. The POC assays enable testing in patient care settings such as doctors’ offices and urgent care facilities. POC tests are one of the urgently needed solutions to address the lab backlogs that are slowing down COVID-19 testing—a critical element in treatment, containment and surveillance.
| US FDA Authorized COVID-19 POC Tests | ||||
| Manufacturer | Test Name | Test Platform | Technology | Testing Time |
| Abbott | ID NOW COVID-19 assay | ID NOW | Isothermal nucleic amplification (Nicking Enzyme Amplification Reaction [NEAR]) | 5 min. and negative results in 13 min. |
| Cepheid (Danaher) | Xpert Xpress SARS-CoV-2 | GeneXpert System | RT-PCR | 45 min. |
| Mesa Biotech | Accula SARS-CoV-2 Test | Accula Dock | PCR | 30 min. |
Cepheid’s Xpert Xpress SARS-CoV-2 assay, Mesa Biotech’s Accula SARS-CoV-2 Test and Abbott’s ID NOW COVID-19 assay gained FDA approval on March 21, March 24 and March 27, respectively. The three tests are authorized under the FDA’s Emergency Use Act (EUA), permitting their use before fulfilling the FDA’s formal and often lengthy approval process for diagnostic tests.
Cepheid, Mesa Biotech and Abbott’s tests utilize nucleic acid amplification technologies (NAAT), as opposed to serological tests, an alternative testing method for POC COVID-19 diagnostic testing. The three tests were developed based on each company’s technology for POC influenza assays, which were all approved under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) waiver process. A CLIA-waved test is “categorized as simple laboratory examinations and procedures that have an insignificant risk of an erroneous result.” In 2015, the FDA waved the first NAAT-based POC test for influenza, providing an enormous boost that market in particular and to POC infectious disease testing in general.
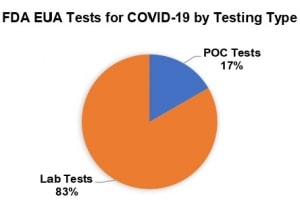
Source: FDA (as of April 1)
According to Kalorama Information’s April 2020 report, “Worldwide Market for Point-of-Care (POC) Diagnostics”, influenza testing accounts for the largest share of the global market for POC infectious disease testing. Abbott is the market leader by far. Other major providers include Quidel and Roche, which like Cepheid and Mesa Biotech, offer NAAT tests.
Just as major providers of POC NAAT tests are now creating a market for COVID-19 assays, leading providers of POC serological tests for influenza testing are seeking to do the same. To do so, BD (Becton, Dickinson and Company), one of the largest providers of POC serological tests for infectious disease, has teamed up with diagnostics developer BioMedomics. Under an agreement announced on March 31, BD is now offering BioMedomics 15-minute, blood-based COVID-19 test. Harry Schein is the test’s exclusive distributor. Although no POC serological test has yet to gain FDA EUA for COVID-19 testing, BioMedomics is seeking approval, according to the North Carolina Biotechnology Center.
However, according to the FDA’s latest guidance, issued on March 16, regarding the serological testing for COVD-19 diagnosis, “Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.”
A complete list of COVID-19 tests, both lab based and POC, authorized under the US FDA EUA is available here.




