SARS-CoV-2 Vaccines: Phase 1 vs. Phase 3
With US regulatory emergency use authorization (EUA) of two vaccines, a major threshold has been crossed in the response to COVID-19. According to the latest information, dated December 16, from the World Health Organization (WHO), more SARS-CoV-2 vaccine candidates are moving closer to completing the clinical trial phase. Twenty percent of the candidates in clinical trials are in Phase 3 trials, or 11 out of 56. And at least 5 have already received EUA from 1 or more countries.
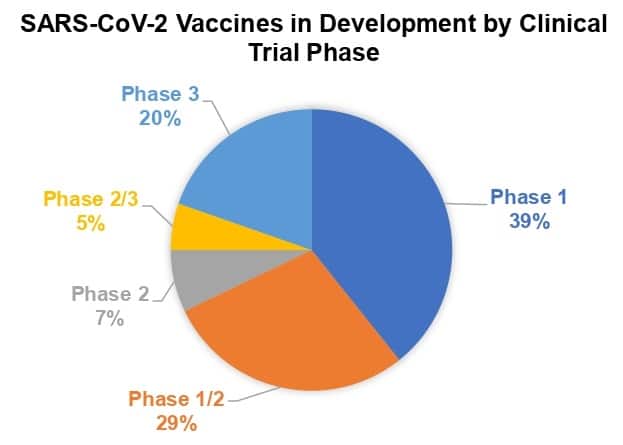
Source: WHO
Pfizer/BioNTech and Moderna’s mRNA vaccines, the first SAR-CoV-2 vaccines approved by the US and also the first mRNA vaccines to ever receive FDA approval, benefitted from timely research and faster production times for mRNA vaccines. But mRNA vaccines are a new technology, with major research advancements dating back to the early 2000s.
However, the largest number of vaccine candidates in Phase 3 belong to more established categories of vaccines: 4 are viral vector (non-replicating)-based vaccines, and 4 are inactivated viruses. The FDA formally approved a viral vector (non-replicating) vaccine for smallpox/monkeypox. Inactivated virus vaccines include polio and hepatitis A.
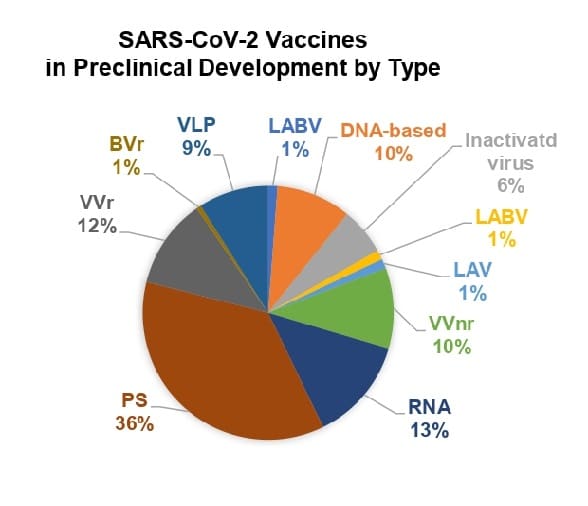
BVr: bacterial vector (replicating) LABV: live attenuated bacterial vector LAV: live attenuated virus PS: protein subunit VLP: virus like particle VVnr: viral vector (non-replicating) VVr: viral vector (replicating)
Source: WHO
In contrast to this first wave of SAR-CoV-2 vaccines, vaccine candidates in Phase 1 clinical trials are more likely to be protein subunit vaccines. This technology accounts for 36% of candidates in Phase 1 out of 22 total. This is also the vaccine technology category showing the greatest increase from Phase 3 to Phase 1. Highly valued for their relative efficiency, protein subunit vaccines also require longer development times than other vaccine processes. In 2019, Merck & Co.’s protein subunit vaccine, Recombivax HB, was FDA approved for hepatitis B.
Not surprisingly, the list of Phase 1 candidates’ vaccine technologies is more diverse. Represented among Phase I candidates but not Phase 3 are DNA, viral vector (replicating), virus like particles, viral vector (nonreplicating) +Antigen Presenting Cell, and live attenuated virus vaccine technology.
Protein subunit vaccines are also the prevalent vaccine type in pre-clinical development, representing 60 out of the total 166 vaccines under development, trailed by RNA vaccines, which total 22. Preclinical vaccine activities also show greater participation by universities, either in partnership with companies or on their own, and international organizations, both public and private. The major pharmaceutical companies with vaccine candidates in preclinical development are Merck & Co. and Sanofi Pasteur, one of the world’s top 3 vaccine makers. Sanofi Pasteur is also working with another of the world’s largest vaccine companies, GSK on a protein subunit vaccine unit now in Phase1-2 trials. Also in Phase 1-2 trials is vaccine candidate from Merck & Co, which is working with Themis, Sharp & Dohme, Institute Pasteur and the University of Pittsburgh.





